Background:
To evaluate the anti-tumor mechanism of IMM47 by blocking the interaction of CD24/Siglec-10 through macrophage antigen presentation.
Methods:
IMM47 was constructed and produced using an in-house-developed CHO-K1 cell expression system. BLI, ELSIA, and flow cytometry methods were used to measure the EC50 of IMM47 binding, ADCC, ADCP, ADCT and CDC activities. hSiglec-10 Tg C57BL/6 mice congenitally transplanted with MC38-hCD24 were used to analyze the in vivo efficacy. IMM47 monotherapy or in combination with SIRP-Fc fusion protein (IMM01) or anti-PD-1 antibodies were used to test IMM47's anti-tumor efficacy in SCID mice with Jeko-1 or MCF-7 tumor cell xenotransplantation and hPD-1 Tg C57BL/6 mice with MC38-hCD24/hPD-L1 tumor cell homologous transplantation models.
Results:
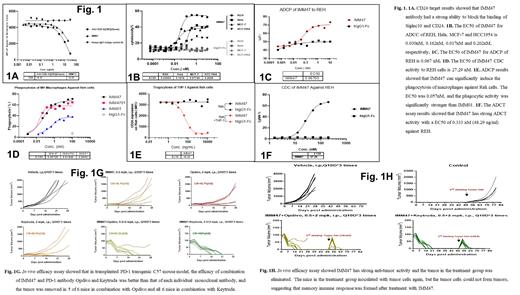
The BLI assay results showed that the affinity between IMM47 and CD24 was 0.876 nM. ELISA results showed that the EC50s of IMM47 binding to CD24 were 0.2847 nM. The EC50 of IMM47 binding to REH, Hela, MCF-7, and HCC1954 cells were 14.09 nM, 7.14 nM, 7.54 nM and 54.89 nM, respectively. IMM47 can specifically bind to CD24-positive cells but not to CD24-negative cells. IMM47 binding to CD24 is independent of the glycosylation modification of the extracellular domain. IMM47 can only bind to CD24 in humans and chimpanzees but not in other species, including mice, rats, pigs, dogs, and cynomolgus. IMM47 does not bind to human erythrocytes but strongly binds to granulocytes. Mechanism of action study results showed that IMM47 blocks the binding of CD24 to Siglec-10, with ADCC EC50 values of 0.030 nM, 0.162 nM, 0.017 nM, and 0.202 nM on REH, Hela, MCF-7, and HCC1954, respectively. IMM47 has strong ADCT activity with an EC50 of 0.333 nM against REH. IMM47 has an ADCP EC50 of 0.067 nM and 0.099 nM on REH and MCF-7, respectively. The phagocytic activity of IMM47 was significantly stronger than that of IMM01, a SIRPα-Fc fusion protein. The CDC activity results showed that the EC50 of IMM47 on REH cells was 27.29 nM.
With hSiglec-10 Tg C57BL/6 mice congenitally transplanted with MC38-hCD24 cells, in vivo efficacy assays showed that IMM47 has strong anti-tumor activity, with higher spleen M1 and M2 immune cells. After the IMM47 treatment, when tumor cells were reinoculated into mice, they did not form tumors, suggesting a memory immune response had been established. After the IMM47 treatment, tumors in 5 of 6 mice were completely cured at a dose of 3 mg/kg, with a dose-dependent relationship being observed. Pharmacodynamic in vivo study in the Jeko-1 tumor cell heterologous transplantation SCID mouse model, IMM47 exhibits strong anti-tumor activity and a dose-dependent relationship. The combination of IMM47 and IMM01 has significantly better efficacy than that of any single drug in the model of MCF-7 xenograft SCID mice. The combination of IMM47 and the PD-1 antibody Tislelizumab in the MC38-hCD24/hPD-L1 tumor cells in the homologously transplanted PD-1 transgenic C57BL/6 mouse model showed the best efficacy, and tumors in all mice were eliminated. The efficacy of IMM47 combined with either the PD-1 antibody Opdivo or Keytruda is better than that of monotherapy, and tumors in 5/6 and 6/6 mice were eliminated when combined with Opdivo and Keytruda, respectively. Four weeks after drug withdrawal, the re-inoculated cells could not form tumors and were eventually eliminated, while the re-inoculated cells in the control group grew normally and formed tumors.
Conclusion:
Our research showed that the humanized anti-CD24 mAb IMM47 had excellent anti-tumor effect. The extracellular domain's N-glycosylation alteration has no effect on IMM47's ability to bind to CD24. The in vitro assays revealed that IMM47 exhibits significant ADCC, ADCP, ADCT, and CDC activities. IMM47 displays strong anti-tumor activity in transgenic mouse models, and a memory immune response is generated after treatment. Additionally, an in vivo pharmacodynamics assay of IMM47 in combination with different PD-1 antibodies revealed that IMM47 exhibits synergistic therapeutic efficacy when combined with Tislelizumab, Opdivo, and Keytruda. This suggests that IMM47 has significant potential for use in the clinical setting for cancer immunotherapy, either alone or in combination with other immune checkpoint inhibitors. Finally, IMM47 avoids the adverse effects displayed by some anti-CD47 antibodies because it does not bind to human red blood cells and has a high safety profile.
Disclosures
Li:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Chen:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Guo:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Yang:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Liu:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Yang:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Bai:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Zhang:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Zhang:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Zhao:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Tu:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Peng:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Liu:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Zhang:ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd: Current Employment. Tian:ImmuneOnco Biopharmaceuticals (Shanghai) Inc: Current Employment, Current equity holder in private company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal